Novel, Rapid Diagnostics for Veterinary Medicine
Novel, Rapid Diagnostics for Veterinary Medicine
Novel, Rapid Diagnostics for Veterinary Medicine
Novel, Rapid Diagnostics for Veterinary Medicine

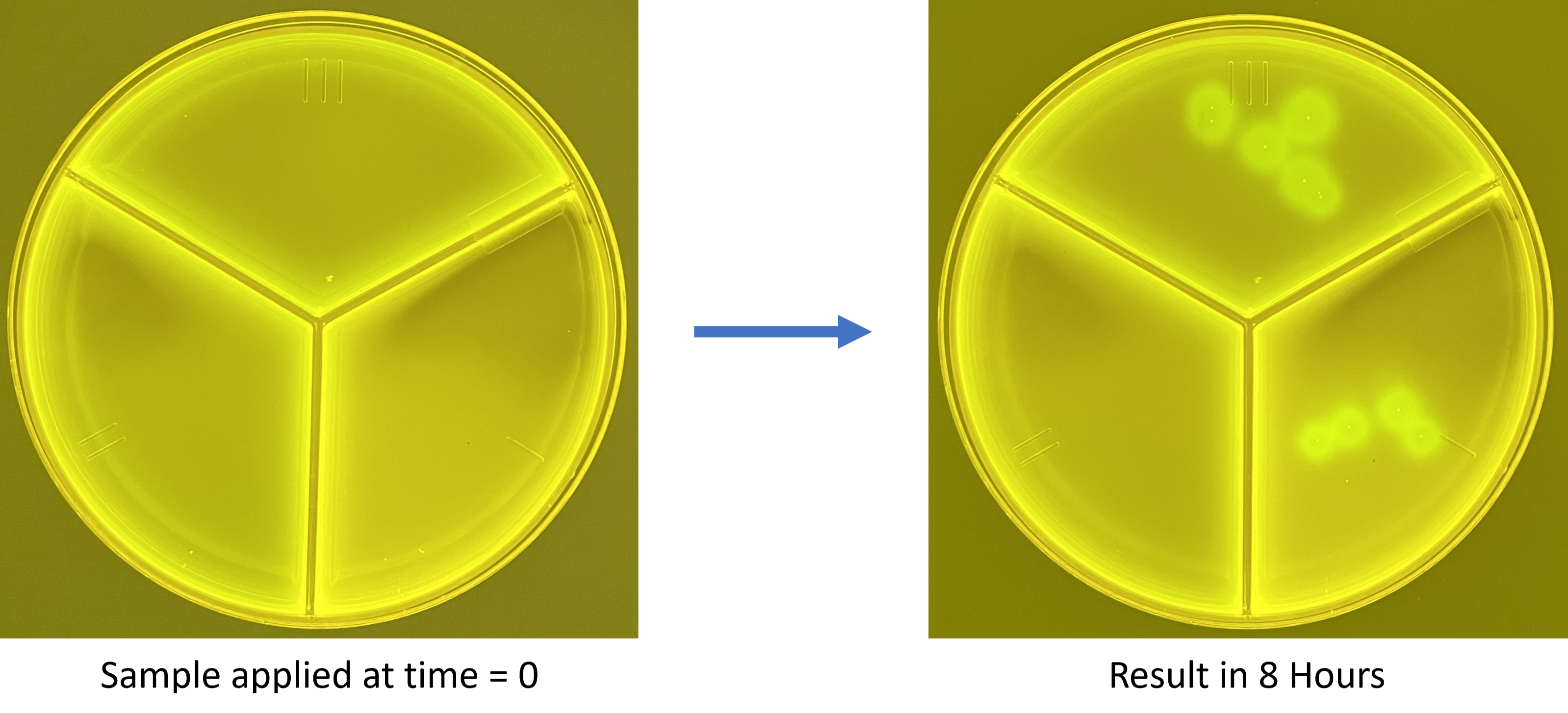
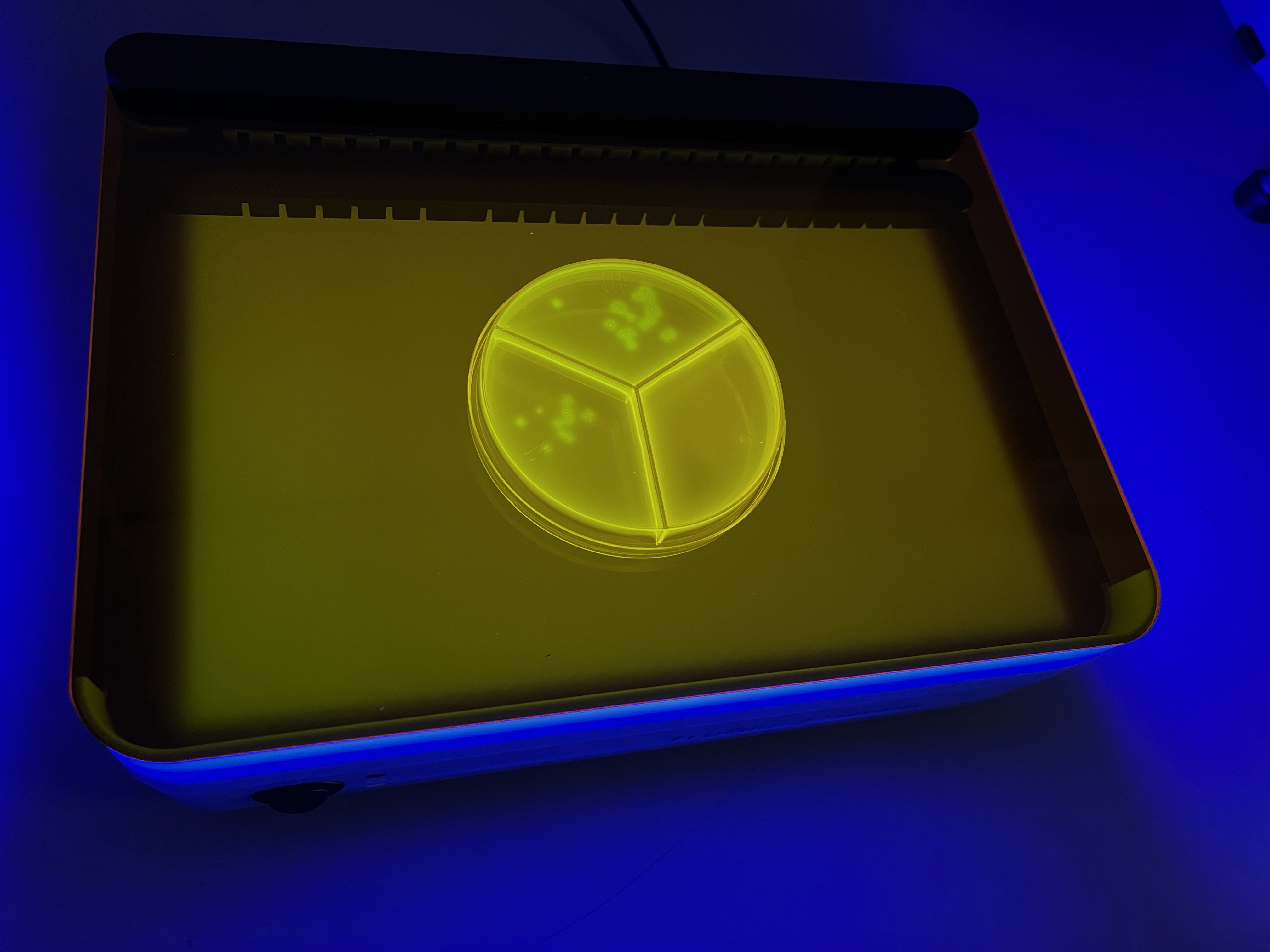
(Laboratory-grown S. pseudintermedius applied to plate. Brightness of images is adjusted.)

(Laboratory-grown S. pseudintermedius applied to plate. Brightness of images is adjusted.)
AVAILABLE PRODUCTS
AVAILABLE PRODUCTS
Don't wait days for Antibiotic Susceptibility Data from Reference Labs
Get Same-Day Results for canine pyoderma with NPT’s NucAP™ Pyoderma Tri-plates!
NucAP™ Pyoderma Tri-plate (Clind/Ox)

Blue light box (Edvotek TruBlu™ 2 Blue/White Transilluminator)
Watch the Full Procedure
More Information
Technology
A simple molecular probe for big infection problems
The Technology
A simple molecular probe for big infection problems
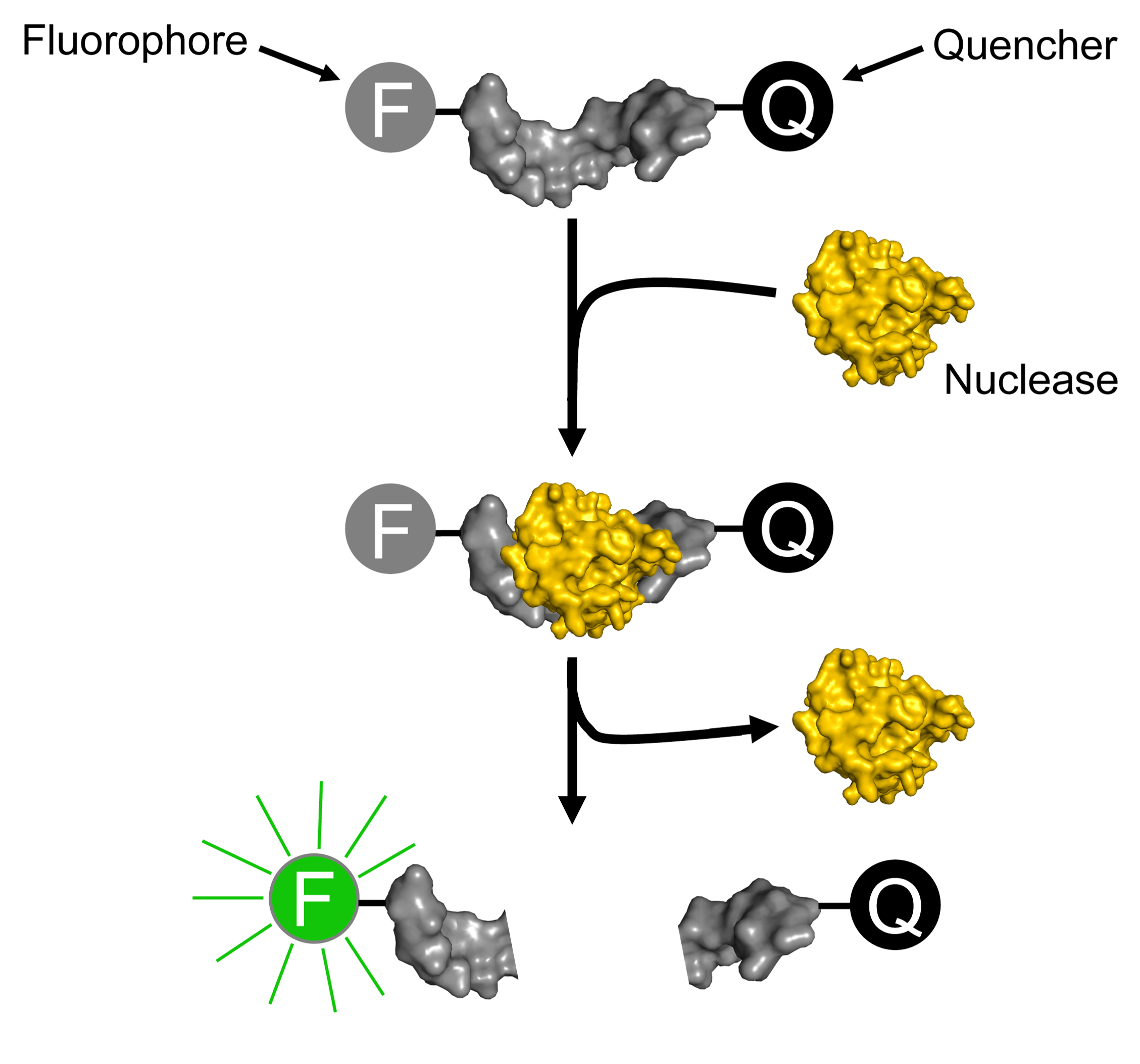
NPT has pioneered a new field of diagnostics that harnesses the enzymatic power of a category of biomarkers known as nucleases. The approach yields uncommon levels of sensitivity and specificity. Because signal generation only occurs if the biomarker is present in the specimen being tested, this approach results in higher signal-to-noise and fewer reagent-dependent artifacts than current methods such as the polymerase chain reaction (PCR) and immunoassays.
The molecular basis of the approach is illustrated in the figure on the right. Each nuclease probe consists of an oligonucleotide (shown in gray) flanked with a fluorophore (F) and a quencher (Q). Upon contact with a disease-associated nuclease biomarker (shown in yellow), the nuclease probe is cleaved, resulting in separation of the quencher from the fluorophore and a consequent increase in fluorescence.
Technical Papers
Applications
Applications
Building a Rich Pipeline
Building a Rich Pipeline
Bloodstream Detection of Pathogens
Bacterial Nucleases Provide Basis For Ultrasensitive Detection
Learn more
Pathogen Detection from Urine
Our technology can be used to identify infectious diseases like urinary tract infections
Learn moreCulture-independent Rapid Detection of Bacterial Pathogens in Blood Samples
Bloodstream infections are responsible for more than 1.5 million patient deaths in the US and worldwide. The rapid detection of bloodstream pathogens and optimization of antibiotic therapy through phenotypic antibiotic susceptibility testing is an important unmet need in critical care medicine and the key to timely intervention and improved outcomes. NPT’s bacterial detection technology has the potential to address the loss of life and cost associated with bloodstream infections. NPT has developed the technical foundation to rapidly detect and determine the antibiotic susceptibility profiles of the deadliest bloodstream pathogens.
Rapid Detection of Urinary Tract Infections
NPT’s technology can be used to rapidly detect other infectious diseases like urinary tract infections (UTI’s). UTI’s are a common infection and antibiotics are often prescribed ‘empirically’ before the results of laboratory tests are available. Current diagnostic methods rely on culture of the specimen, identification of bacteria responsible for the infection, and determination of antibiotic susceptibility. This process often takes days. The NucAP™ technology has been applied to rapidly detect urinary tract infections and determine the antibiotic susceptibility profile of the causative pathogens in hours, providing a potential solution for an important unmet need.
Detection of Circulating Tumor Cells
In proof-of-concept work, the nuclease technology has been used to detect circulating tumor cells isolated from the blood of late-stage breast cancer patients. This demonstration suggests the technology could provide a sensitive tool for monitoring treatment response or detecting disease recurrence in cancer patients, or perhaps even a sensitive screening tool for the early detection of cancer.
About NPT
Nuclease Probe Technologies was founded to solve one of the most basic problems in diagnostics—the need to rapidly detect diseases. Early detection enables veterinarians and physicians to make informed treatment decisions faster and achieve better outcomes for patients.

James founded NPT to commercialize rapid diagnostic applications based on the nuclease detection technology platform invented in his academic research program, and he has served as CEO since 2018. He is an inventor on several pending and issued patents that cover the platform and has authored twenty-five peer-reviewed scientific publications, including 8 studies involving the nuclease detection technology. His pursuit of the nuclease detection platform has been supported by over $2M in grants. James recruited a team of subject matter experts who provide NPT with complementary business and scientific expertise to develop the commercial potential of the technology. Since opening NPT’s R&D operation in the M2D2 startup incubator in Lowell, Massachusetts in 2019, James has overseen the development and commercialization of NPT’s NucAP™ Pyoderma Tri-plate, a nuclease detection-based veterinary diagnostic. James previously earned a BS in Chemical Engineering at the University of Virginia, a PhD in Neurobiology at Duke University and completed a post-doctoral fellowship in RNA-based therapeutics at Duke. Prior to opening NPT’s Massachusetts operations, he was a tenured Associate Professor in the Department of Internal Medicine at the University of Iowa.

Blake W. Buchan, Ph.D.— Associate Director, Clinical Microbiology, Medical College of Wisconsin

Dr. Buchan is in charge of the education of residents in the Pathology residency program at MCW. He is the Associate Residency Program Director for Clinical Pathology, Course Director for the PGY-1 Molecular Pathology rotation, and attending faculty for the PGY-2 Microbiology rotation. In these roles he leads trainees in practical, didactic, and discussion sessions related to education in clinical microbiology and molecular diagnostics. Dr. Buchan serves in the clinical role of Associate Director for Clinical Microbiology and Molecular Diagnostics in which he oversees many aspects of the clinical microbiology laboratory including test interpretation, review of daily results, and council of technologists. In addition, he serve as Principal Investigator for numerous clinical trials involving new diagnostic assays for the identification of bacterial, viral and fungal pathogens

Dr. Bradley Ford, MD PhD, is Director of Microbiology at the University of Iowa Hospitals and Clinics and a Member of the American Society of Microbiology, as well as a member of the Editorial Board of the Journal of Clinical Microbiology. Dr. Ford is an author of over 53 peer-reviewed journal publications. In his role as Director of Microbiology at the University of Iowa Hospitals and Clinics, Dr. Ford is responsible for all patient testing, training of physicians and laboratory staff, and oversight of microbiology laboratory operations. Dr. Ford has evaluated and led the adoption of new microbiological methods for pathogen identification and antibiotic susceptibility testing including novel molecular devices. Dr. Ford has served as the principal investigator on projects that evaluated the clinical efficacy of new methods for rapid identification and antibiotic susceptibility testing of bacteria present in blood cultures, as well as, other molecular methods for identifying genetic mechanisms of antibiotic resistance in bacteria.
Senior Executive Advisor — Philippe Nore

Philippe is an experienced Startup CEO in the Life Sciences industry with a passion for driving the adoption of new products and technologies. Prior to NPT, he co-founded and led as CEO for several years MiNDERA, a MedTech company developing Next-Generation Skin Diagnostics & Analytics. Prior to MiNDERA Philippe was Senior International Business Leader at Roche Diagnostics where he held global P & L responsibility and launched several FDA-approved products. Philippe has held prior business consulting roles in the life sciences industry with Bain & Company a global management consulting firm, and for LEK Consulting, a world renown management and strategy consulting firm. Philippe is a proven general manager and business development expert in the Life Sciences industry with extensive US and international experience. Philippe has a demonstrated track record of developing and launching new businesses and a passion for driving the adoption of new products and technologies. Philippe holds a MSc. in chemistry from ESPCI (Paris) and an MBA from the Wharton School where he graduated as a Palmer Scholar.
Nuclease Probe Technologies is building a new class of diagnostic tools that harnesses the enzymatic potential of protein biomarkers to solve major problems in veterinary medicine and healthcare. We are looking for passionate, driven individuals who are interested in joining us in this pursuit. If this sounds like you, please drop us a note; we’d love to hear from you!
Nuclease Probe Technologies, Inc. is proud to be an Equal Opportunity Employer. Our goal is to have a diverse workforce. We do not discriminate on the basis of race, age, color, religion, national origin, gender, sexual orientation, gender identity or expression, veteran status or disability or any other status protected under federal, state or local law. All employment is decided on the basis of qualifications, merit, and business need.
Patents
United States patents licensed by Nuclease Probe Technologies include: 9,603,949; 10,619,219; 10,653,800; 11,155,882. Additional US patents owned or licensed by Nuclease Probe Technologies are pending.
United States patents licensed by Nuclease Probe Technologies include: 9,603,949; 10,619,219; 10,653,800; 11,155,882. Additional US patents owned or licensed by Nuclease Probe Technologies are pending.
News
Nuclease Probe Technologies raises $500,000 from Breakout Labs to Support Development of Rapid Antibiotic Susceptibility Testing for Bloodstream Infections
Nuclease Probe Technologies raises $500,000 from Breakout Labs to Support Development of Rapid Antibiotic Susceptibility Testing for Bloodstream Infections
March 5, 2019 08:00 AM Eastern Daylight Time
Lowell, MA—Nuclease Probe Technologies, Inc., a company pioneering a precision medicine
approach to matching patients with the most effective antibiotics to treat deadly bloodstream
infections, announces the investment by Breakout Labs...
Read More
Contact Us
Nuclease Probe Technologies, Inc.
110 Canal Street, 4th FloorFor business development or other inquiries please contact bd@nptrapidtesting.com.